

ABSTRACT
Background: Syphilis is a chronic systemic sexually transmitted infection that affects tens of millions of people annually. At the anorectal level, its polymorphic manifestation requires differential diagnosis with benign and malignant anorectal diseases.
Objective: To describe the different presentation of anorectal syphilis from 5 clinical cases.
Methods: Observational, retrospective, descriptive study.
Results: Most of the patients were HIV positive in sexually active age. The manifestations recorded, like those reported in the literature, were fissures, perianal ulcers and pseudotumors.
Conclusions: Syphilis is considered “the great pretender.” In the anorectal location, a high diagnostic suspicion is required to differentiate it from similar presentations of other benign anal diseases, inflammatory bowel disease and anorectal cancer, in order to avoid the consequent risk of overtreatment. Syphilis is considered “the great pretender.” In the anorectal location, a high diagnostic suspicion is required to differentiate it from similar presentations of other benign anal diseases, inflammatory bowel disease and anorectal cancer, in order to avoid the consequent risk of overtreatment.
Keywords: anorectal syphilis, anal fissure, luetic ulcer, pseudotumor
Syphilis is a chronic systemic disease, with asymptomatic periods.1 Its etiological agent is the spirochete Treponema pallidum, identified at the end of 1905 due to the difficulty of its isolation.2 It was baptized as syphilis in 1530 by the Italian poetry of the pastor Syphilis. It is also known as the French disease: Morbus gallicus or Neapolitan disease due to the epidemic among the soldiers of King Charles VIII at the end of the 15th century during the siege of Naples.2 However, far from being a historical disease, it is a current issue. Globally, it causes the disease in tens of millions of people per year, and an increase in the number of cases is currently documented.3 In Uruguay, it has been a notifiable disease since 2008, with a prevalence of 98.6 cases per 100,000 inhabitants.4 It is a sexually transmitted infection. As such, it is preventable, but at the same time stigmatizing, which often delays diagnosis. Its systemic dissemination explains the florid clinical manifestations, which leads to considering it as "the great pretender".5 At the anorectal level, its manifestation is polymorphic; includes fissures, ulcers, proctitis, and pseudotumors with regional lymph node involvement. This particularity makes it a differential diagnosis of benign and malignant anorectal diseases.
A review of the clinical presentations of anorectal syphilis was carried out from a series of clinical cases.
In this retrospective, observational study, patients diagnosed with anorectal syphilis treated at the General Surgery Polyclinic in 2020 were included. The variables gender, age, HIV infection, risky sexual behavior, and clinical presentation were recorded. Data from medical records and imaging studies were obtained with the prior informed consent of the patients
Five patients were treated, 3 men (2 HIV-positive) and 2 women (1 HIV- positive), aged between 32 and 40 years. The presentation was as anal fissure, perianal ulcers and pseudotumors.
- A 32-year-old woman, HIV negative, sex worker, consulted for anal pain of 3 months' evolution. Anoperineal inspection revealed multiple deep perianal fissures at hours 10, 4, and 6 (Fig. 1 A).
The diagnosis of perianal syphilis was confirmed with VDRL. Treatment with intramuscular benzathine penicillin G (BPG) was performed with complete remission of symptoms.
- A 40-year-old male, without risky sexual behavior consulted for 15 days of pain due to anal fissure at hour 7 (Fig. 1 B and C). The diagnosis of perianal syphilis was confirmed with VDRL. Treatment was performed with intramuscular BPG. The diagnosis of perianal syphilis was confirmed with VDRL. Treatment with intramuscular benzathine penicillin G (BPG) was performed with complete remission of symptoms.
- A 40-year-old male, without risky sexual behavior consulted for 15 days of pain due to anal fissure at hour 7 (Fig. 1 B and C). The diagnosis of perianal syphilis was confirmed with VDRL. Treatment was performed with intramuscular BPG.
- A 35-year-old male, HIV-positive, with good immune status and undetectable viral load, consulted for multiple painful perianal lesions. Anoperineal inspection revealed the presence of irregular ulcers at the anal margin (Fig. 2). The VDRL test was reactive. Treatment was performed with intramuscular BPG.

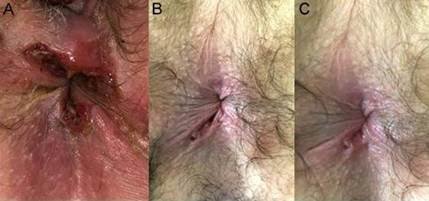
Figure 1. Luetic anal fissures. A. Multiple deep fissures. B. Single fissure before treatment.C. Single fissure after treatment.
 |
Figure 2. Multiple luetic perianal ulcers. A. Before treatment. B. After treatment with benzathin penicillin G.
- A 32-year-old woman, HIV-positive, with unknown immune status, allergic to penicillin and with unprotected anoreceptive sex, consulted for a 1 month history of a painful raised perianal lesion. Perineal inspection confirmed the presence of an indurated and painful tumor at the anterior anal margin (Fig. 3 A). The VDRL test was reactive. Doxycycline was prescribed with complete remission of symptoms.
- A 40-year-old male, HIV- positive with good immune status and undetectable viral load and no risky sexual
behavior, consulted for rectal syndrome. Digital rectal examination revealed an ulcerated lesion on the anterior aspect, 1 cm from the anorectal ring, suggestive of malignancy. Sigmoidoscopy confirmed a proliferative, vegetative and ulcerated lesion. MRI showed a circumferential tumor of the lower rectum, with mesorectal and extra-mesorectal adenophaties, probably T3N1 (Fig. 3 B). The pathological anatomy diagnosed a non-specific inflammatory lesion, suspicious of rectal syphilis. The diagnosis was confirmed with serology. After treatment with intramuscular BPG he had complete clinical and endoscopic remission.
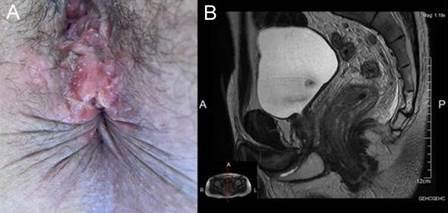
Figure 3. Luetic anal and rectal pseudotumor. A. The elevated lesion is observed on the anterior anal margin. B. MRI, sagittal section, shows circumferential thickening of the subperitoneal rectum, reported as a probable T3N1 middle rectal tumor.
The incidence of anorectal syphilis is unknown, since there are no official reports that discriminate by anatomical location. From the bibliographic review, most of the publications correspond to case reports (Table 1). Case series are rare. Among them, we highlight one from Argentina (whose population could share the epidemiological profile with that of Uruguay), published by Cipollone et al. in 2019,6 reporting a total of 77 patients treated in 5 years. In this series, 61 patients were men, 12 cis women, and 4 trans women. The mean age was 30 years (19-72).
Anorectal syphilis occurs more frequently in sexually active men (Table 1). Although they are most often homosexual men or trans women, the risk is independent of sexual orientation. Population studies reflect an increase of anal intercourse in heterosexuals in last decades. 7 The prevalence increases in HIV-positive patients, who share the risk factors of STIs.1
The prevalence of co-infection between syphilis and HIV varies between 45 and 79%.8 Syphilis acts as a facilitator of transmission, due to the disruption of the epithelial/mucosal barrier and local inflammation. The activation of the immune response in infected hosts contributes to an increase in viral replication.8
Syphilis occurs in two stages: early and late. Early syphilis includes primary, secondary, and latent syphilis. Latent syphilis constitutes the asymptomatic period.9 Primary syphilis is the initial stage that manifests itself with the typical chancre. It appears from 10 to 90 days after sexual contact. It is classically described as a single, painless superficial ulcerated lesion that may be associated with regional lymph node involvement. Anorectal chancre is particularly difficult to diagnose, as it may go unnoticed by the patient. It is often very painful and multiple.8 The differential diagnosis should be made with the Herpes Virus.9 At this stage, serology can be negative or indeterminate, so it is recommended to repeat it at 1, 2, and 6 weeks in order to exclude the diagnosis. However, this behavior delays treatment and can be risky in patients who do not comply with follow-up or who have difficulties accessing the health system. Even without treatment, the chancre resolves spontaneously and may remain without an etiological diagnosis, which explains the underestimation of cases. Secondary syphilis develops in approximately one third of untreated patients. It includes contagious and systemic skin manifestations secondary to treponemal dissemination within the first two years of infection.9 In anal location it can give rise to fissures, ulcers and flat warts. If it manifests as a single fissure, the symptoms are similar to conventional anal pathology and diagnosis is difficult. It is not uncommon for patients to receive topical or even surgical treatments. The lesion will disappear even without specific treatment in a variable period of 1 to 6 weeks, which can generate a false sense of healing.7
Luetic ulcers are usually multiple, often with irregular edges. The facing arrangement is classic, known as the “kiss” configuration.10 Although the manifestation as a flat wart was not recorded in our series, some authors describe it in 6 to 23% of patients. It consists of well-defined, friable, and painful hypertrophic plaques that occur in intertriginous areas. The differential diagnosis should be made with HPV infection or malignant tumors.7 Rectal manifestations may be proctitis, ulcerated lesions, or pseudotumors with involvement of the mesorectal lymph nodes. The most consistent feature is ulceration. Some authors suggest that all anogenital ulcers should be considered syphilitic until proven otherwise.9 Symptoms are nonspecific and include bleeding, tenesmus, urgency, and purulent, mucoid, or bloody anal discharge. They may erroneously lead to the diagnosis of lymphogranuloma venereum (an entity that may coexist), inflammatory bowel disease, or malignant neoplasms if ulcerated lesions are detected on rectal
examination. Localized lesions also support differential diagnosis with solitary ulcer syndrome or lymphoma. Patients with secondary syphilis frequently present systemic signs such as rash on the trunk, palms, and soles, fever, anorexia, headache, arthralgia, and hepatosplenomegaly.9 The disappearance of the lesions does not imply healing, since even without treatment this will occur after weeks to months, beginning the latency period. Late syphilis includes neurosyphilis, cardiovascular syphilis, and gummatous syphilis.9
The diagnosis of syphilis depends on the demonstration of treponemas (direct test) or serological tests (indirect).1 Direct visualization of Treponema pallidum is rare. Diagnosis is by indirect methods. These include serologic tests for nonspecific (nontreponemal) and specific (treponemal) antibodies. Nontreponemal tests include VDRL (venereal disease research laboratory) and RPR (rapid plasma reagin). Highly sensitive and not very specific, they are used as detection and follow-up methods.9 Treponemal tests, which include FTA (Fluorescent Treponemal-Antibody Absorption) and TPHA (Treponema Pallidum Haemogglutination Assay), are highly specific (95-98%). They are used to confirm a positive nontreponemal test and remain positive for life.4,9 Negative serology does not exclude the diagnosis, and its repetition is necessary. In addition, serologies for other STIs should be requested.
Sigmoidoscopy, indicated when there is suspicion of anorectal involvement, allows magnified visualization of the mucosa and biopsy of suspicious lesions. MRI is requested in the context of rectal lesions. It is common to report the presence of regular thickening of the rectal wall, associated with adenopathies and changes in mesorectal fat.12-15 These findings are indistinguishable from carcinoma, except that there is no diffusion restriction.5
In most cases, histopathology shows elements of non- specific chronic inflammation.11-16 The concomitant finding of intraepithelial neoplasias is not uncommon, especially in HIV-positive patients,11 requiring appropriate evaluation and treatment.
Treatment of syphilis is simple, with widely available and inexpensive antibiotics. The World Health Organization and the 2020 European guidelines for early syphilis recommend the administration of a single dose of 2.4 million units of intramuscular BPG. On the other hand, in cases of uncertain duration, as happens to be the majority, they recommend three weekly doses.1,9
Alternative treatment for patients allergic to penicillin consists of doxycycline 200 mg/day orally for 14 days for early syphilis and 21 to 28 days for late latent syphilis.9 There are other specific plans for neurosyphilis, ocular and atrial syphilis, and pregnant patients.
Upon completion of treatment, clinical and serological evaluation should be performed at 6 and 12 months. The patient's sexual contacts should be traced, reported, and treated prophylactically.1
In this series, anorectal syphilis occurred more frequently in HIV-positive patients of active sexual age. The manifestations recorded, like those reported in the literature, were fissures, perianal ulcers and pseudotumors.
It is essential to take a complete history of risky sexual behaviors and request serology to rule out other STIs. Given the polymorphic clinical manifestations secondary to its early systemic dissemination, syphilis is considered "the great pretenderr".
At the anorectal level, a high diagnostic suspicion should be taken in the face of presentations similar to usual anal pathology, inflammatory bowel disease, and anorectal cancer, to avoid the consequent risk of overtreatment.
Table 1. Review of the literature on case reports of anorectal syphilis.
|
Author |
Age |
Sex |
HIV |
Symptoms and signs |
SIG |
Histopathology |
Imaging |
Diagnosis |
|
López 2018 |
48 |
M |
No |
Tenesmus and rectal bleeding |
Irregular ulcers |
Acute rectitis. Spirochetes + |
WD |
WD |
|
Díaz-Jaime 2017 |
35 |
M |
Yes |
Rectal bleeding |
Irregular ulcerated lesion |
Plasma cell infiltration and cryptitis. Spirochetes + (W-S) |
WD |
Reactive RPR and TPHA |
|
Pisani 2015 |
48 |
M |
Yes |
Rectal bleeding |
Ulcerated lesion |
Spirochetes + (W-S) |
MRI: Thickened rectal wall (4-6 cm) and mesorectal fat stranding |
WD |
|
Shu 2014 |
32 |
M |
No |
Rectal bleeding and mass |
Tumoración vegetante |
Acute rectitis |
ERUS: T3N1 |
Reactive TPHA |
|
García Robles 2013 |
33 |
M |
Yes |
Proctitis |
Irregular ulcerated lesions |
Chronic rectitis with areas of acute inflammation and glandular obstruction with submucosal involvement |
CT: Thickening of the rectal wall, perirectal fat infiltration and necrotic regional adenopathies |
Reactive RPR and TPHA |
|
Cha 2010 |
45 |
M |
No |
Proctitis, tenesmus and rectal bleeding |
Ulcerated lesion |
Chronic rectitis. Spirochetes + (W-S) |
CT and MRI: Parietal thickening with perirectal fat infiltration and mesorectal adenopathies |
Reactive RPR and TPHA |
|
Wentao 2010 |
48 |
M |
No |
Rectal bleeding and mass |
Ulcerated lesion |
Infiltration of plasma cells and lymphocytes |
CT: Thickening of the rectal wall |
Positive TPHA |
|
Fabbraro 2008 |
34 |
M |
No |
Rectal bleeding |
Ulcerated lesion |
Infiltration of plasma cells and lymphocytes. Dark field: Spirochetes + |
CT: Bilateral iliac lymph nodes |
Reactive VDRL 1/83 |
|
Caselli 2009 |
37 |
M |
Yes |
Proctitis and mass |
Ulcerated lesion |
Anal intraepithelial neoplasia II |
MRI: Infiltrative transmural lesión, mesorectal and external iliac adenopathies |
Reactive RPR and TPHA |
|
Furman 2008 |
28 |
M |
Yes |
Proctitis and rectal bleeding |
Ulcerative proctitis |
Chronic rectitis. Spirochetes + (W-S) |
WD |
Reactive RPR and FTA |
SIG: Sigmoidoscopy. WD: Without data. W-S: Warthin-Starry stain. RPR: Rapid plasma reagin. TPHA: Treponema pallidum haemogglutination assay. MRI: magnetic resonance imaging. ERUS: Endorectal ultrasonography. CT: Computed tomography. VDRL: Venereal disease research laboratory. FTA: Fluorescent treponemal-antibody absorption.
1. Justin D. Radolf, Edmund C. Tramont y Juan C. Salazar. En: Mandell, Douglas y Bennett. Enfermedades infecciosas. Principios y práctica. Sífilis (Treponema pallidum). 2017. Tomo III. Cap 237. pag 2865-92.
2. Turnes, A. La sífilis en la medicina: una aproximación a su historia. Montevideo: Ediciones Granada. 2007.
3. Fenton KA, Breban R, Vardavas R, Okano JT, Martin T, Aral S, et al. Infectious syphilis in high-income settings in the 21st century. Lancet Infect Dis. 2008; 8:244-53.
4. Ministerio de Salud Pública. Recomendaciones de diagnóstico, tratamiento y vigilancia de las infecciones de transmisión sexual. Uriguay. 2010.
5. Pisani Ceretti A, Virdis M, Maroni N, Arena M, Masci E, Magenta A, Opocher E. The great pretender: Rectal syphilis mimic a cancer. Case Rep Surg. 2015; 2015:434198.
6. Cipollone S, Svidler López L, López Aquino D, Sidra G, Cabrini M, Ventura M, et al. Sífilis anorrectal: Una entidad subdiagnosticada. Casuística de un hospital público de la Ciudad
Autónoma de Buenos Aires. ASEI Actualizaciones en sida e infectología. 2019; 27:66-73.
7. Svidler L, La Rosa L. Relato oficial: infecciones transmisibles sexualmente que afectan colon, recto y ano. El rol del cirujano entre mitos y tabúes. Rev Argent Coloproct. 2022; 33 (4): 4-97.
8. Svidler L. Manifestaciones coloproctológicas de las infecciones de transmisión sexual ocasionadas por chlamydia trachomatis, neisseria gonorrhoeae y treponema pallidum. Presentación casuística. Rev Argent Coloproct. 2019; 30: 80-7.
9. Janier M, Unemo N, Dupin G, Tiplica M, Potocnik, Patel R. 2020 European guidelines on the management of syphilis. J Eur Acad Dermatol Venereol. 2021; 35: 574-88.
10. Furman DL, Patel SK, Arluk GM. Endoscopic and histologic appearance of rectal syphilis. Gastrointest Endosc. 2008; 67: 161, commentary 161-2.
17. ry syphilis. Dig Liver Dis. 2008; 40:579-81.
11. Caselli G, Pinedo G, Niklistschek S. Tumor rectal como presentación de sífilis primaria. Rev Chil Cirug. 2009; 61:290-93.
12. Pruzzo R, Redondo F, Amaral H, Glasinovic E, Caviedes I, Glasinovic JC. Anal and rectal syphilis on F-18 FDG PET/CT. Clin Nucl Med. 2008; 33:1-4.
13. Jae Myung C, Sung C, Joung L. Rectal syphils mimicking rectal cancer. Yonsei Med J. 2015; 51:276-78.
14. Díaz-Jaime F, Satorres-Paniagua C, Bustamante-Balén M. Primary chancre in the rectum: An underdiagnosed cause of rectal ulcer. Rev Esp Enferm Dig. 2017; 109: 236-37.
15. Zheng S, Jing-long Z, Xiao-Fei D, Chen-Jin G, Feng S, Chi-shing
Z. Primary chancre in the rectum: a report of rare case of syphilis. Radiol infect Dis. 2014; 1:29e31.
16. Febbraro A, Manetti G, Balestrieri P, Zippi M. Rectal cancer or rectal chancre? Beware of prima